Abstract
Background: Hematopoietic cell transplantation associated thrombotic microangiopathy (TA-TMA) shares common features with atypical hemolytic uremic syndrome (aHUS) and thrombotic thrombocytopenic purpura. TA-TMA affects multiple organ systems resulting in multi-organ failure and non-relapse mortality (NRM). Recently, complement activation has been associated with TA-TMA leading to utilization of complement blockade in TMA. Eculizumab is a humanized monoclonal antibody that binds to C5 and prevents the formation of the membrane attack complex (C5b-9) or the terminal complement pathway. Eculizumab may be an effective treatment for pediatric TA-TMA patients; however, there are limited data regarding its use in adult TA-TMA patients. Furthermore, eculizumab is an expensive medication and there are no published data regarding the financial implications of TA-TMA treated with eculizumab. The purpose of this study was to examine the response rates, NRM, and financial implications of TA-TMA treated with eculizumab.
Methods: We performed a retrospective cohort study of patients with TA-TMA who received eculizumab at our institution between January 1, 2012, and April 9, 2017. TA-TMA was defined according to the BMT-CTN criteria with evidence of hemolysis, elevated LDH, renal dysfunction and negative Coombs' test. Serum complement biomarkers (C5b-9) were used to support the diagnosis when platelet or red blood cell transfusion dependence occurred with organ dysfunction or tissue evidence of microangiopathy. Patients were vaccinated against Neisseria meningitides and received appropriate antibiotic prophylaxis. Eculizumab therapy was initiated at the Food and Drug Administration-approved dosing schedule for aHUS: 900 mg intravenously weekly for the first 4 weeks followed by 1200 mg intravenously every other week beginning on week 5 for a total of 8 weeks, with some patients requiring longer than an 8 week course. The primary outcome was complete response to eculizumab therapy and responders were defined by achievement of transfusion independence and improvement in creatinine. Secondary outcomes included non-relapse mortality (NRM), inpatient cost, number of comorbidities, number of infections, number of hospitalizations, and hospital length of stay (LOS). Comorbidities were defined as any documented medical problem other than the reason for transplant and TA-TMA.
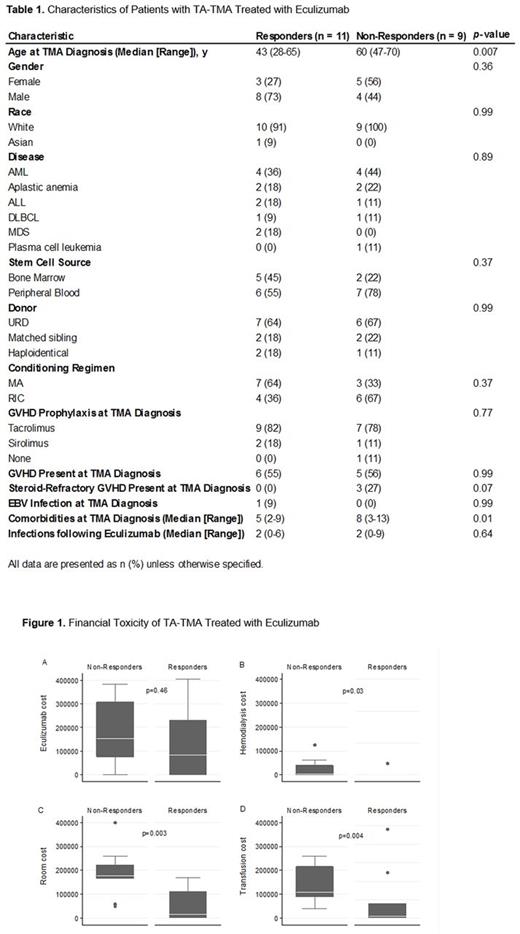
Results: Twenty patients were included in the study (Table 1). The median (range) time from transplant to diagnosis of TA-TMA was 139 (14-388) days. The median (range) time from diagnosis of TA-TMA to first dose of eculizumab was 2 (0-272) days. The complete response rate was 55%. The median (range) number of doses of eculizumab was 6.5 (2-17) for responders compared to 3 (1-7) for non-responders including both inpatient and outpatient doses (p= 0.01). There were no significant differences between the number of hospitalizations for responders and non-responders. However, responders had shorter LOS and lower inpatient costs than non-responders. The median (range) LOS was 9 days (0-92) for responders compared to 61 days (26-94) for non-responders (p= 0.03). The median (range) total inpatient cost was $259,734 (0-1,670,070) for responders compared to $1,525,758 (385,092-1,948,190) for non-responders (p= 0.003). The itemized inpatient costs are shown in Figure 1. Patients who responded to eculizumab tended to be younger with less comorbidities. In fact, patients were 33% less likely to respond to eculizumab for every additional documented comorbidity at the time of TA-TMA diagnosis (HR=0.67 [95% CI 0.49-0.92]). The NRM was 1/11 (9%) for responders and 9/9 (100%) for non-responders (HR=0.07 [95% CI: 0.007-0.66]).
Conclusions: TA-TMA is an important complication of HCT that is associated with high NRM and financial toxicity. Our data suggests that patients who respond to eculizumab have lower NRM, LOS, and financial costs than non-responders. There was a statistically non-significant trend in incidence of steroid-refractory GVHD in the non-responder group; additionally non-responders received significantly fewer doses than responders, which may explain partly the lower response rates compared to the 80% response rates in aHUS. Patient age and the number of comorbidities at the time of diagnosis of TA-TMA may be important predictive factors for response to eculizumab.
Mims: Novartis: Honoraria. Hofmeister: Janssen: Research Funding; Karyopharm: Research Funding; Celgene: Research Funding; Thrassos: Honoraria, Membership on an entity's Board of Directors or advisory committees; Bristol-Myers Squibb: Research Funding; Adaptive Biotechnologies: Honoraria, Membership on an entity's Board of Directors or advisory committees; Roche: Research Funding; Takeda: Research Funding. Walker: Gilead: Research Funding. Vasu: Stemline Therapeutics: Research Funding; Boehringer-Ingelheim: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal